By Carole Novielli
 The FDA has approved a generic version of the abortion pill Mifeprex, at a time when more deaths are being reported and the abortion industry is pushing for the dangerous abortion inducing chemicals to be dispensed online or by mail. According to a notice from the FDA, the generic version is approved for use as an abortifacient. This news comes just a day before the FDA updated its adverse effects reports through 2018, stating, “As of December 31, 2018, there were reports of 24 deaths of women associated with Mifeprex since the product was approved in September 2000, including two cases of ectopic pregnancy resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal…” The FDA’s 2017 report put the number of deaths at 22.
The FDA has approved a generic version of the abortion pill Mifeprex, at a time when more deaths are being reported and the abortion industry is pushing for the dangerous abortion inducing chemicals to be dispensed online or by mail. According to a notice from the FDA, the generic version is approved for use as an abortifacient. This news comes just a day before the FDA updated its adverse effects reports through 2018, stating, “As of December 31, 2018, there were reports of 24 deaths of women associated with Mifeprex since the product was approved in September 2000, including two cases of ectopic pregnancy resulting in death; and several cases of severe systemic infection (also called sepsis), including some that were fatal…” The FDA’s 2017 report put the number of deaths at 22.
This means that in 2018 alone, two more women died from taking the abortion pill. And yet, now a generic version is going to be made available.
To date, the report documents nearly 4,200 reported adverse effects, including hospitalization and other serious complications.
On April 11, 2019, the FDA approved GenBioPro, Inc.’s abbreviated new drug application for a generic Mifeprex, which, when used with Misoprostol is approved as an abortion pill regimen. The FDA states (emphasis added), “This approval reflects FDA’s determination that GenBioPro’s product, Mifepristone Tablets, 200 mg, is therapeutically equivalent to Mifeprex and can be safely substituted for Mifeprex. Like Mifeprex, the approved generic product is indicated for the medical termination of intrauterine pregnancy through 70 days gestation….”
READ: The secrecy surrounding the abortion pill’s maker and influential financial investors must end
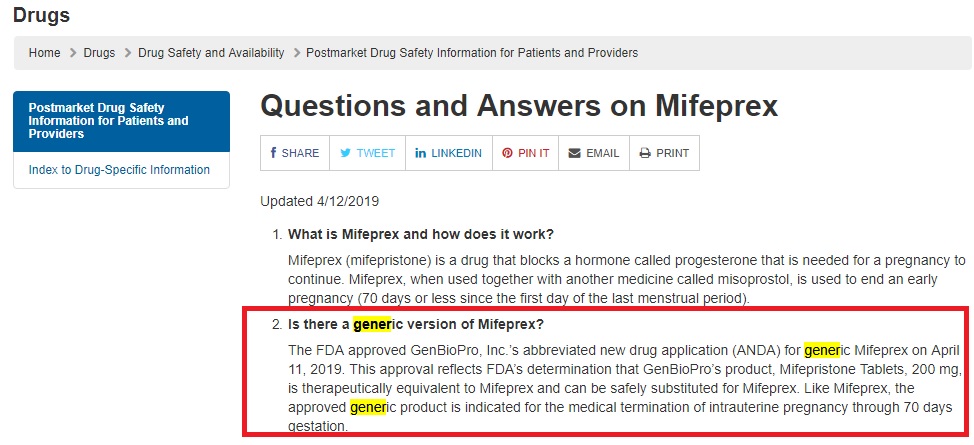
Generic abortion pill by GenBioPro approved by FDA
The FDA also states clearly that although they modified the Mifeprex application to include “mifepristone products,” this change in no way removes the FDA’s REMS (Risk Evaluation and Mitigation Strategy), “a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use.”
Of note, on April 11, 2019, FDA approved a supplemental application for Mifeprex, approving modifications to the existing approved REMS for Mifeprex to establish a single, shared system REMS for mifepristone products (including Mifeprex as well as the approved generic version of Mifeprex) for the medical termination of intrauterine pregnancy through 70 days gestation. In establishing the single, shared system REMS, no changes were made to the substantive elements of the REMS. This single, shared system REMS is known as the Mifepristone REMS Program.
Find approval information for this 2019 supplement here.
The approved generic version of Mifeprex generally has the same labeling as Mifeprex…Under the law, the approved generic version of Mifeprex is required to use a single, shared system REMS with the brand product, Mifeprex. This single, shared system REMS, known as the Mifepristone REMS Program, sets forth the distribution requirements that must be followed for both Mifeprex and the approved generic version of Mifeprex.
Requiring that the generic be subject to FDA’s REMS is good news for now, because, as Live Action News has previously reported, the abortion lobby is attempting to expand access to the abortion pills via mail order or pharmacy by pushing “self-managed abortion,” described by Guttmacher as ending a pregnancy “without direct supervision by a health care provider.” To accomplish this, REMS, must be eliminated.
READ: What you should know about the dangerous ‘self-managed’ abortion pill push
Dr. Donna Harrison, Executive Director American Association of Pro-Life Obstetricians and Gynecologists(AAPLOG), agreed, telling Live Action News (emphasis added):
Allowing a generic equivalent of Mifeprex basically means that the patent restrictions have run out. So, new drugs have a patent which is time limited… and in the case of Mifeprex, the patent was held by the Population Council. So, now that the patent has run out, generics are allowed. But this does not change the restrictions. REMS are still in place until FDA changes the restrictions. So, just to clarify, this does not mean that Mifeprex is OTC [over-the-counter]. It just changes how much quality control goes in to the pill manufacturing process and how much Danco can charge for the drug.
The move towards a generic drug is being hailed a victory by abortion advocates, specifically Dr. Daniel Grossman, who has deep ties to the “self-managed” abortion pill push.
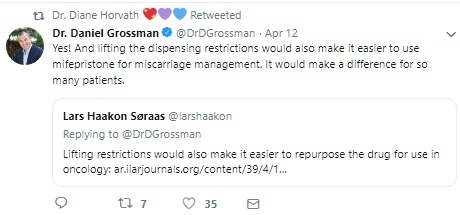
FDA Approves generic abortion drug (Image Twitter Dr. Daniel Grossman)

FDA Approves generic abortion drug NWHC (Image: Twitter)
Under the Mifepristone REMS Program, the FDA states, “Mifeprex and the approved generic version of Mifeprex” may:
- [O]nly be supplied directly to healthcare providers who are certified to prescribe the drug product and who meet certain qualifications.
- [T]he products are only available to be dispensed in certain healthcare settings, specifically, clinics, medical offices and hospitals, by or under the supervision of a certified prescriber.
- They are not available in retail pharmacies and are not legally available over the Internet.
The original drug (Mifeprex) was approved in 2000 after being brought to the U.S. by the eugenics-founded Population Council, and was seeded by the Packard Foundation among other pro-abortion philanthropy groups. They then set up a highly secretive company named Danco to manufacturer the drug.
The generic version is produced by GenBioPro, Inc., which also appears to have the financial supportof abortion collaborators. In fact, Packard gave GenBioPro, Inc. $185,000 in 2016 and 100,000 in 2017. According to the Nevada Secretary of State, a 2007 filing for the company was permanently revoked. A new filing in 2011 is active and shows a registered agent of CSC Services of Nevada, Inc.and the only officer listed is E. Masingill.
The FDA warns consumers they should not buy Mifeprex or GenBioPro, Inc.’s approved generic version of Mifeprex, Mifepristone Tablets, 200 mg., over the Internet because they will bypass important safeguards designed to protect patient health.
This entry was posted
on Wednesday, April 17th, 2019 at 4:49 pm and is filed under News & Commentary.
You can follow any responses to this entry through the RSS 2.0 feed.
You can skip to the end and leave a response. Pinging is currently not allowed.
As more women die from abortion pill, the FDA approves a generic version
By Carole Novielli
This means that in 2018 alone, two more women died from taking the abortion pill. And yet, now a generic version is going to be made available.
To date, the report documents nearly 4,200 reported adverse effects, including hospitalization and other serious complications.
On April 11, 2019, the FDA approved GenBioPro, Inc.’s abbreviated new drug application for a generic Mifeprex, which, when used with Misoprostol is approved as an abortion pill regimen. The FDA states (emphasis added), “This approval reflects FDA’s determination that GenBioPro’s product, Mifepristone Tablets, 200 mg, is therapeutically equivalent to Mifeprex and can be safely substituted for Mifeprex. Like Mifeprex, the approved generic product is indicated for the medical termination of intrauterine pregnancy through 70 days gestation….”
READ: The secrecy surrounding the abortion pill’s maker and influential financial investors must end
Generic abortion pill by GenBioPro approved by FDA
The FDA also states clearly that although they modified the Mifeprex application to include “mifepristone products,” this change in no way removes the FDA’s REMS (Risk Evaluation and Mitigation Strategy), “a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use.”
Of note, on April 11, 2019, FDA approved a supplemental application for Mifeprex, approving modifications to the existing approved REMS for Mifeprex to establish a single, shared system REMS for mifepristone products (including Mifeprex as well as the approved generic version of Mifeprex) for the medical termination of intrauterine pregnancy through 70 days gestation. In establishing the single, shared system REMS, no changes were made to the substantive elements of the REMS. This single, shared system REMS is known as the Mifepristone REMS Program.
Find approval information for this 2019 supplement here.
The approved generic version of Mifeprex generally has the same labeling as Mifeprex…Under the law, the approved generic version of Mifeprex is required to use a single, shared system REMS with the brand product, Mifeprex. This single, shared system REMS, known as the Mifepristone REMS Program, sets forth the distribution requirements that must be followed for both Mifeprex and the approved generic version of Mifeprex.
Requiring that the generic be subject to FDA’s REMS is good news for now, because, as Live Action News has previously reported, the abortion lobby is attempting to expand access to the abortion pills via mail order or pharmacy by pushing “self-managed abortion,” described by Guttmacher as ending a pregnancy “without direct supervision by a health care provider.” To accomplish this, REMS, must be eliminated.
READ: What you should know about the dangerous ‘self-managed’ abortion pill push
Dr. Donna Harrison, Executive Director American Association of Pro-Life Obstetricians and Gynecologists(AAPLOG), agreed, telling Live Action News (emphasis added):
Allowing a generic equivalent of Mifeprex basically means that the patent restrictions have run out. So, new drugs have a patent which is time limited… and in the case of Mifeprex, the patent was held by the Population Council. So, now that the patent has run out, generics are allowed. But this does not change the restrictions. REMS are still in place until FDA changes the restrictions. So, just to clarify, this does not mean that Mifeprex is OTC [over-the-counter]. It just changes how much quality control goes in to the pill manufacturing process and how much Danco can charge for the drug.
The move towards a generic drug is being hailed a victory by abortion advocates, specifically Dr. Daniel Grossman, who has deep ties to the “self-managed” abortion pill push.
FDA Approves generic abortion drug (Image Twitter Dr. Daniel Grossman)
FDA Approves generic abortion drug NWHC (Image: Twitter)
Under the Mifepristone REMS Program, the FDA states, “Mifeprex and the approved generic version of Mifeprex” may:
The original drug (Mifeprex) was approved in 2000 after being brought to the U.S. by the eugenics-founded Population Council, and was seeded by the Packard Foundation among other pro-abortion philanthropy groups. They then set up a highly secretive company named Danco to manufacturer the drug.
The generic version is produced by GenBioPro, Inc., which also appears to have the financial supportof abortion collaborators. In fact, Packard gave GenBioPro, Inc. $185,000 in 2016 and 100,000 in 2017. According to the Nevada Secretary of State, a 2007 filing for the company was permanently revoked. A new filing in 2011 is active and shows a registered agent of CSC Services of Nevada, Inc.and the only officer listed is E. Masingill.
The FDA warns consumers they should not buy Mifeprex or GenBioPro, Inc.’s approved generic version of Mifeprex, Mifepristone Tablets, 200 mg., over the Internet because they will bypass important safeguards designed to protect patient health.
This entry was posted on Wednesday, April 17th, 2019 at 4:49 pm and is filed under News & Commentary. You can follow any responses to this entry through the RSS 2.0 feed. You can skip to the end and leave a response. Pinging is currently not allowed.